The Chartis® System
Accurately Predict Responders to Zephyr® Valve Treatment
The Chartis System* has been used in multiple randomised clinical trials1-4 to predict likely responders to the Zephyr Valve treatment. It provides precise flow and pressure readings for specific lobes in the lung to assess absence of collateral ventilation, which is a predictor of response to treatment.
*The Chartis System includes the Chartis Console and Catheter.
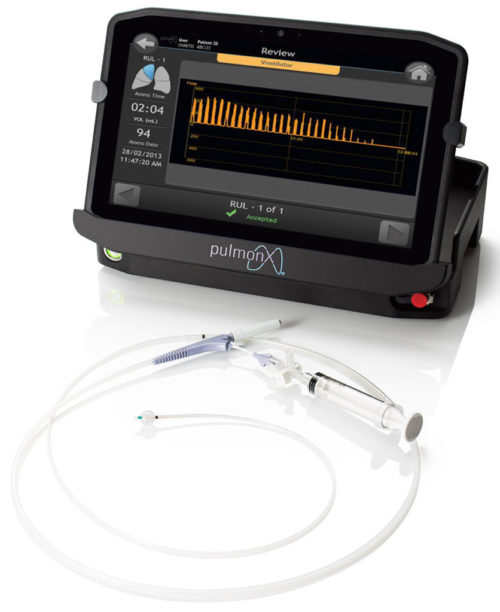
Overview of the Chartis Tablet Features

Touchscreen
12.5″ wide viewing angle display. Full HD (1920 x 1080) graphics with “View Anywhere®” outdoor 1000 nit sunlight readable display
Viewing Flexibility
The tablet display can be moved or viewed independent of its base, which houses the interface with the Chartis catheter, to maximise utilisation of space in the operating room and support simultaneous bronchoscope and Chartis visualisation

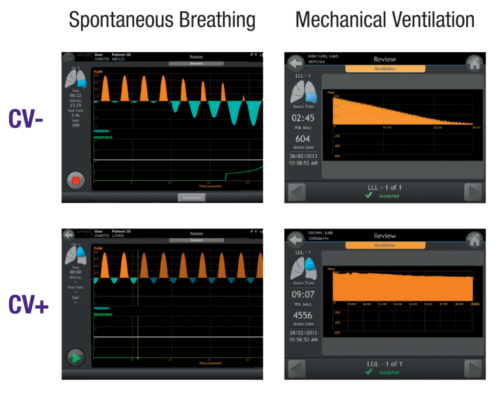
Multiple Ventilation Modes
Works with spontaneous breathing or mechanical ventilation

Password Protected Log in and Secure Data Storage
Offers superior protection that can support hospital administration and PHI (protected health information) compliance considerations

Bluetooth® Connectivity
Allows the tablet display to be positioned in easy view and reach
In patients who have been assessed by the Chartis System as having low collateral ventilation, treatment with Zephyr Endobronchial Valves lead to high responder rates and clinically significant improvement in symptoms compared to Standard of Care (SOC).1
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.
View Anywhere® is a registered trademark owned by Zebra Corp.
Bluetooth® is a registered trademark owned by Bluetooth SIG, Inc.
Chartis System Enables Detection of Collateral Ventilation Under Different Sedation Methods
Chartis System Ordering Information*
| Description | Catalog Number |
| Chartis Pulmonary Assessment System – Console | CHR-CO-300 |
| Chartis Catheter | CHR-CA-12.0 |


