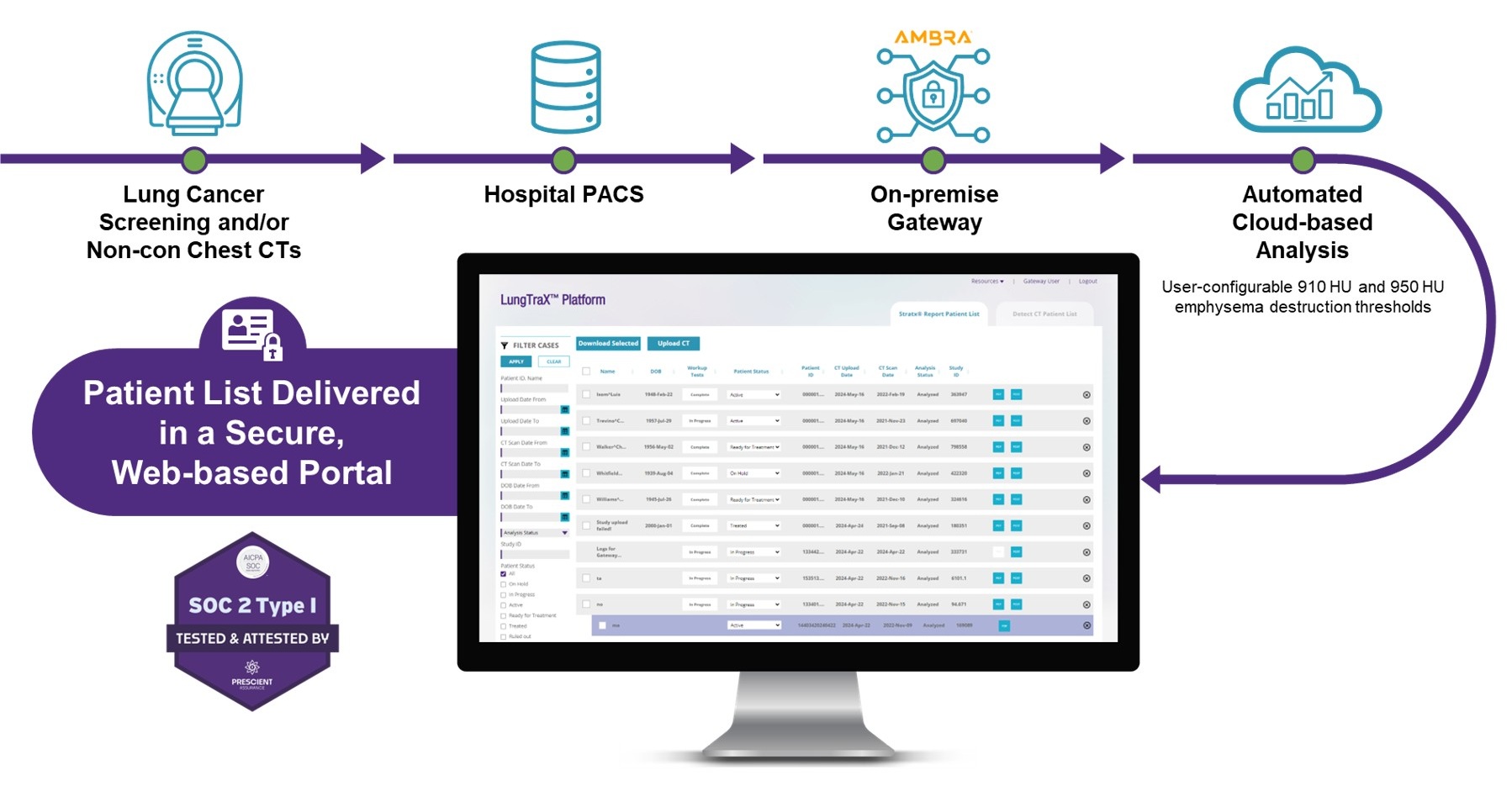
LungTraX™ Platform
The LungTraX Platform offers a comprehensive solution to enhance and expand your Zephyr® Valve program. This PACS-integrated system facilitates automated patient identification, collaborative workup management, and treatment planning with StratX® Reports—all within a single web-based platform.
LungTraX Connect
LungTraX Connect Software enables a streamlined, efficient, and collaborative workup process to allow more time for delivering valuable care to patients.
Key Features
1. PACS Integration
LungTraX Connect Software leverages an existing partnership with Ambra® Health to offer a seamless PACS integrated CT scan upload solution, enabling the direct transfer of CT scans for StratX Lung Analysis Reports.

2. Protected Health Information (PHI) Enabled
LungTraX Connect Software automates the retrieval of patient name and date-of-birth directly from the CT scan to display in the Case List in a secure and HIPAA compliant manner. There is no need to keep track of patient IDs in a separate document.
3. Collaborative Patient Management Tools
The easy-to-use workup test tracker, along with the open notes functionality, empowers your team by providing a way to visualize the next steps required in determining patient candidacy for Zephyr Valves.
4. Serves as the Connection-Point for Additional Integration Tools
LungTraX Detect CT
LungTraX Detect CT automates the identification of underdiagnosed patients with severe COPD/ emphysema in a way that seamlessly integrates into your Zephyr Valve program workflow.
Key Features
1. Automated Patient Identification*
- Prospectively analyzes non-contrast chest CTs to identify patients within your hospital who have radiographically-significant emphysema.
- LungTraX Detect found between 10.5% to 18% of lung cancer screening CT scans had high level of emphysema2.
2. Easy-to-Use Patient Management Tools
- Protected health information (PHI) enabled web portal provides tools to organize and manage patient candidates for Zephyr Valve treatment.
- Track PFT, comorbidity, smoking status, and access ordering physician information for easy outreach.
3. LungTraX Portal Integration
- Integration into the LungTraX Portal makes it easy to request StratX® Lung Analysis Reports and seamlessly transition a patient into the Zephyr Valve workup process.
* Pulmonx’s LungTraX Detect CT and StratX Reports help present information from CT scans analyzed by FDA-cleared software (Thirona’s LungQ, K232412) for quantitative assessment of lung structure and function to support clinical decision making, diagnosis and follow-up. LungTrax Detect CT assists healthcare providers with visualizing this LungQ data, which may suggest the presence of emphysema. Refer to Thirona’s indications for use here: https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K232412
How CT-based Identification Works with LungTraX Detect CT
Presence of Emphysema in the National Lung Cancer Screening Trial1

Presence of Valve-eligible Emphysema in a Pulmonx LungTraXTM Detect Pilot2 Pulmonx found:
| Center | LDCTs Analyzed | Valve-eligible Emphysema (%) |
| Center 1 | 256 | 10.5% |
| Center 2 | 275 | 18.1% |
| Center 3 | 609 | 15.2% |
1. Pinsky PF, Lynch DA, Gierada DS. Incidental Findings on Low-Dose CT Scan Lung Cancer Screenings and Deaths From Respiratory Diseases. Chest. 2022;161(4):1092-1100.
2. Identifying Undiagnosed Emphysema: Utility of Automated QCT Software for CT Screening. Ma, Kevin et al. CHEST, Volume 166, Issue 4, A6478 – A6479.
Platform Configurations
| Feature | Standard LungTraX Platform | LungTraX Connect | LungTraX Detect CT |
| Manual CT upload | ✔ | ✔ | ✔ |
| Access to StratX baseline and post-treatment reports | ✔ | ✔ | ✔ |
| Access to basic web portal | ✔ | ||
| PACS-integrated CT transfer for baseline reports | ✔ | ✔ | |
| PHI-enabled web portal | ✔ | ✔ | |
| Collaborative patient management tool | ✔ | ✔ | |
| Upgradeable for future connected products and services | ✔ | LungTraX Connect subscription required | |
| CT-based population screening | ✔ |


