Clinical Study Summaries

FEV1 Percent Change
from Baseline to 12 Months
Published in The American Journal of Respiratory and Critical Care Medicine1
The benefits are comparable to those seen with LVRS (lung volume reduction surgery) but with a reduction in post-procedure morbidity.”
- Multicenter, multinational, randomized controlled trial
- Results out to 12 months
- Heterogeneous emphysema with little to no collateral ventilation
- Clinically meaningful and statistically significant benefits over current standard of care medical therapy
- Improvements in:
- Lung function
- Exercise tolerance
- Dyspnea
- Quality of life

Improvement in lung function measured by FEV1
(Change from Baseline to Follow-up Time point)
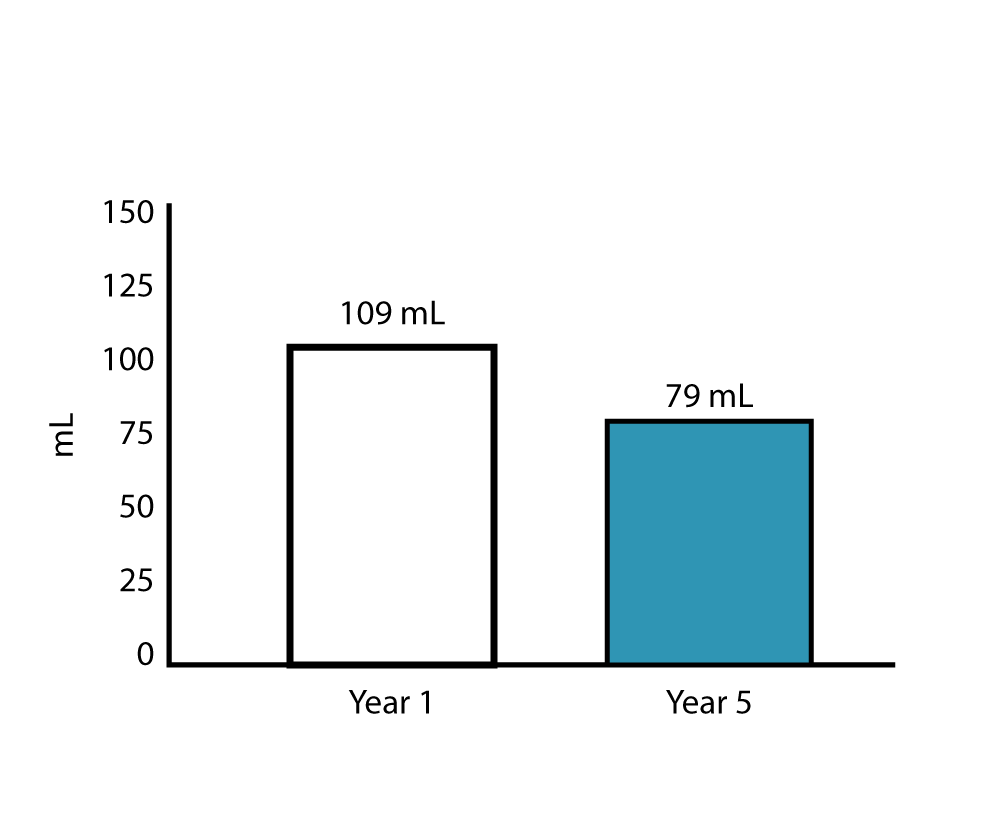
The LIBERATE study demonstrates durable improvements in lung function for patients treated with the Zephyr Endobronchial Valve. This is the longest follow-up of Zephyr Valve treated patients out to 5-years in a prospective study
- Annual improvements in lung function measured by FEV1 ranging from 109 mL in Year 1 to 79 mL at Year 5, with an acceptable safety profile
- FEV1 improvement over baseline through year 5 is considered an advantage over maximal medical treatment alone given the known decline in lung function over time
- Similar or lower incidence of respiratory adverse events or serious adverse advents (SAEs) through Year 2 to Year 5 compared to Year 1 post-procedure
- No new types or increase in frequency of respiratory SAEs compared to prior years

Severe COPD Exacerbations

LIBERATE post-hoc analysis on lobar volume reduction of ≥50% influence on severe exacerbation frequency at 12 months.
- Subjects with TLVR ≥50% had significantly better clinical outcomes
- Severe COPD exacerbation rate was significantly reduced compared to the SoC (n=62) during the 46 day to 12-month post-EBV period

FEV1 Percent Change
from Baseline to 6 Months
Published in The American Journal of Respiratory and Critical Care Medicine2
Benefits are in line with those seen with LVRS (lung volume reduction surgery), and the consistent trial results, potential reduction in post-procedure morbidity, and reversibility of the procedure position Zephyr Valve treatment as a viable treatment option in those who remain symptomatic on maximal medical therapy.”
- Multicenter, multinational, randomized controlled trial
- Heterogeneous emphysema with little or no collateral ventilation
- Clinically meaningful and statistically significant benefits over current standard of care medical therapy
- Improvements in:
- Lung function
- Exercise tolerance
- Dyspnea
- Quality of life

FEV1 Percent Change
from Baseline to 6 Months
Published in The American Journal of Respiratory and Critical Care Medicine3
EBV therapy in selected patients with homogeneous emphysema without collateral ventilation results in clinically meaningful benefits of improved lung function, exercise tolerance, and quality of life. Given the very limited treatment options available for this patient population, EBV therapy should be considered in these patients.”
- Prospective randomized controlled trial
- Homogeneous emphysema with little or no collateral ventilation
- Clinically meaningful benefits in:
- Lung function
- Exercise tolerance
- Quality of life

FEV1 Percent Change
from Baseline to 6 Months
Published in The New England Journal of Medicine4
Endobronchial valve treatment in patients with emphysema and a proven absence of interlobar collateral ventilation provided a measurable clinical benefit, with significantly improved lung function, exercise capacity, and quality of life as compared with usual care.”
- Prospective randomized controlled trial
- First randomized study to use Chartis® System to pre-select candidates
- Emphysema with little or no collateral ventilation
- Statistically and clinically greater improvements than the control group for:
- Pulmonary function (FEV1 and FVC)
- Exercise capacity
- Quality of life
- One-year follow-up published in The American Journal of Respiratory and Critical Care Medicine5

