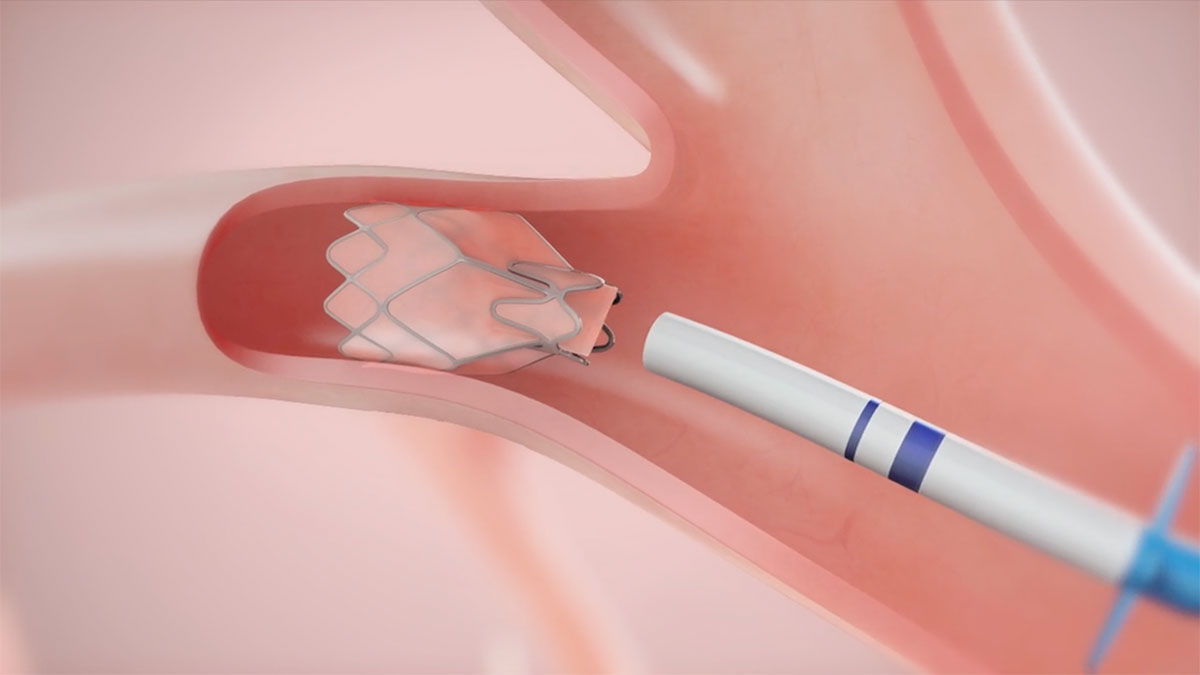
Breakthrough Technology for Severe COPD/Emphysema
The Zephyr Valve is a clinically-proven bronchoscopic treatment for patients with severe COPD/emphysema – without surgery and its associated risks.1-5
Zephyr Valves: Most Studied. Most Proven. Most Used Valves for Severe COPD/Emphysema1
- Demonstrated clinically meaningful benefits in lung function, exercise capacity, and quality of life (QoL).1
- Clinically-proven, safe and effective treatment for patients with severe emphysema
- Consistent clinical findings across four randomised control trials

Programs & Webinars
Access a library of peer-to-peer and CME resources created by leading medical societies, pulmonologists, and Medscape.