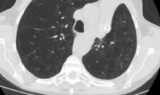
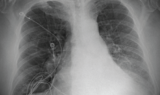
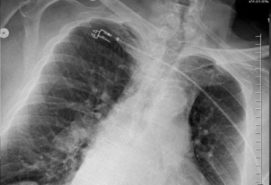
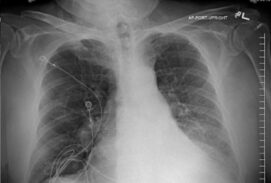
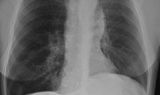
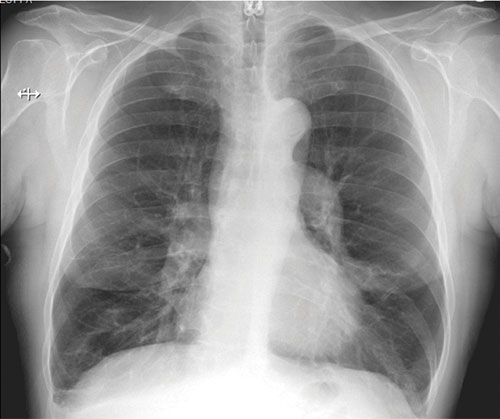
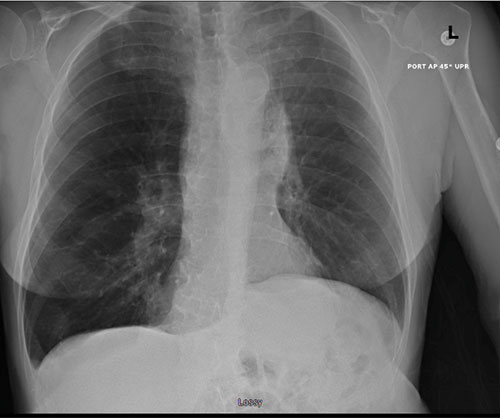
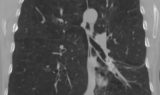
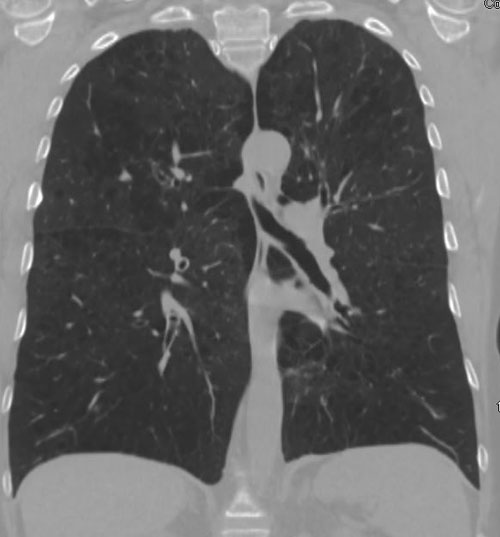
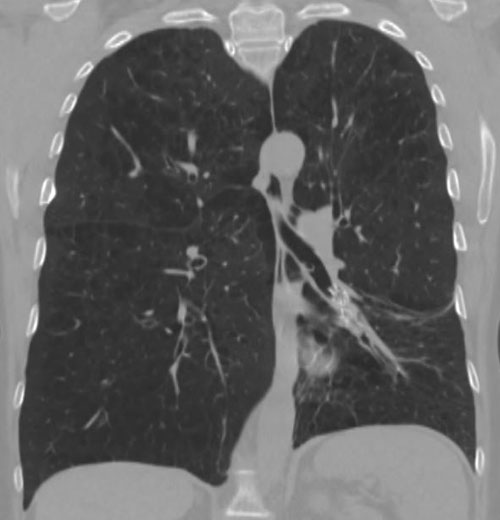
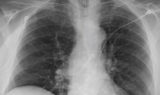
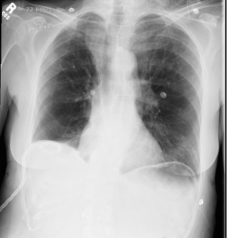
62-year-old female with marked reduction in volume in the treated lung
Clinical Spotlight
62-year-old female with marked reduction in volume in the treated lung
| Patient Profile | |
|---|---|
| Quality of Life Goal | Wants to be more active, keep up with kids and grandkids |
| Condition |
|
| Smoking History |
|
| Management Medications |
|
| Oxygen Use |
|
| Baseline FEV1 |
|
| Symptoms |
|
| Special Notes |
|
Procedure Details:
-
Pulmonary rehab prior to treatment
-
Collateral ventilation negative (confirmed with Chartis®) System
-
Four Zephyr® Valves placed in left upper lobe (LUL)
-
Patient tolerated the procedure well, no adverse events
-
Three-night hospital stay for monitoring
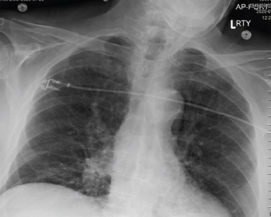
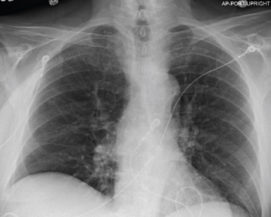
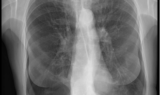
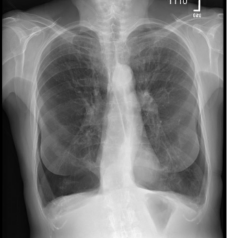
Pre and Post-Procedure Imaging:
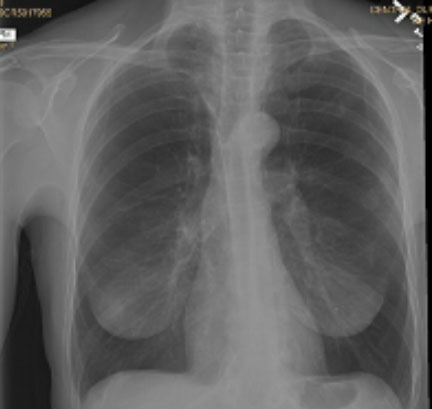
Pre-procedure
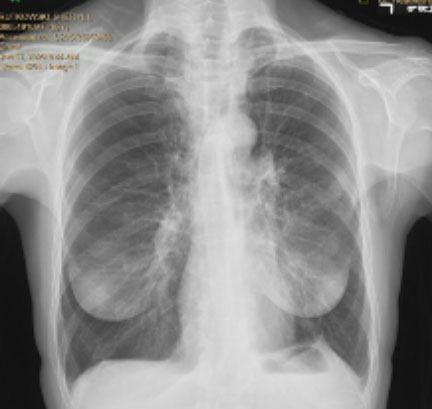
Post-procedure
Pre and Post-Procedure Lung Function Chart:
| ASSESSMENT | PRE-PROCEDURE | POST-PROCEDURE (3 MONTHS) |
|---|---|---|
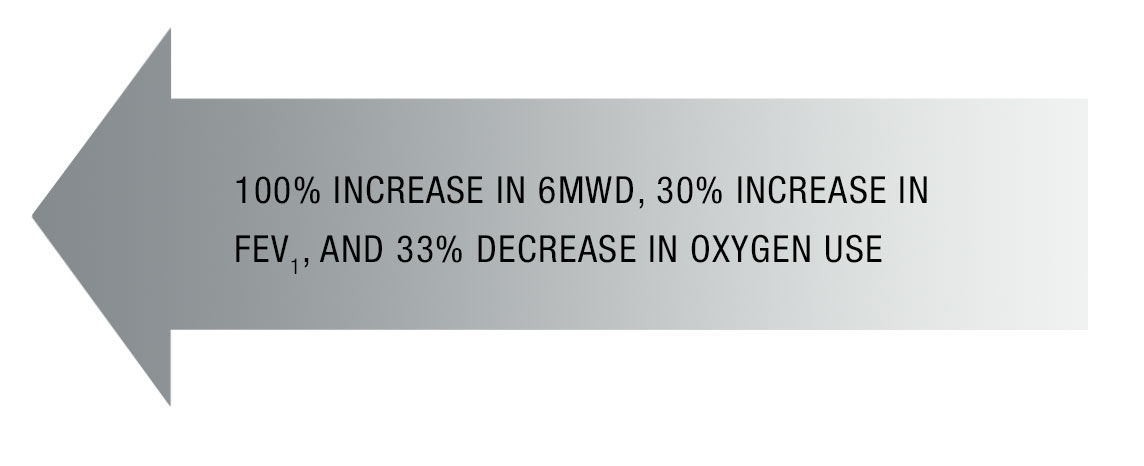
| FEV1 | 0.68 mL | 1.04 mL |
| FEV1 (% pred) | 32% | 49% |
| RV | 584 mL | 358 mL |
| RV (% pred) | 386% | 237% |
| TLC | 786 mL | 568 mL |
| TLC (% pred) | 195% | 141% |
| DLCO (% pred) | 40% | 45% |
| 6MWD | 335 m | 457 m |
Case was Completed by:
Dr. Benjamin Seides at Central DuPage Hospital, Winfield, IL
Patient was Referred by:
Dr. Linda M. Lam (Pulmonary, Critical Care)
Patient Outcome:
Patient had excellent response with:
-
Marked improvement in exertional capacity, including long walks
-
No hospitalizations since treatment
-
Breathing is easier, less shortness of breath
-
Remains on daily maintenance medications
-
Overall improvement in quality of life
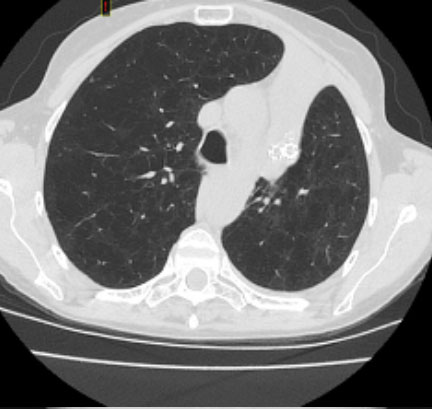

How the Zephyr Valve Works:
The Zephyr Valve is a one-way valve designed to reduce hyperinflation of the lungs caused by severe emphysema/COPD. In a minimally invasive bronchoscopic procedure, an average of four tiny valves are placed in the airways to block off the diseased parts of the lungs where air gets trapped, causing hyperinflation and severe shortness of breath. The Zephyr Valve reduces lung hyperinflation by allowing trapped air to escape and preventing new air from entering that diseased lobe. This allows the healthier parts of the lung to function better and results in patients being able to breathe more easily and experience less shortness of breath.1
The Zephyr Endobronchial Valve is removable, and thus preserves future therapy options.
If you have a patient to refer, find a treatment center in your area.
Zephyr Valve Patient Benefits and Risks:
Patients treated report significant improvements in lung function, exercise tolerance, and quality of life.1
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.
Results from clinical spotlights are not necessarily predictive of results in other cases. Results in other cases may vary.
GLO-EN-674-v2
Important Safety Information: The Pulmonx Zephyr Endobronchial Valves are implantable bronchial valves indicated for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation. The Zephyr Valve is contraindicated for: Patients for whom bronchoscopic procedures are contraindicated; those with evidence of active pulmonary infection; known allergies to Nitinol (nickel-titanium) or its constituent metals (nickel or titanium); known allergies to silicone, or with large bullae encompassing greater than 30% of either lung; Patients who have not quit smoking. The Zephyr Valve should be used with caution and only after careful consideration in treating patients with: Prior lung transplant, LVRS, median sternotomy, or lobectomy; Congestive heart failure or recent myocardial infarction; FEV1 <15% of predicted value. Use is restricted to a trained physician. Prior to use, please reference the Zephyr Endobronchial Valve System Instructions for more information on indications, contraindications, warnings, all precautions, and adverse events. Caution: Federal law restricts this device to sale by or on the order of a physician.