Endobronchial-valve treatment in patients with emphysema and a proven absence of interlobar collateral ventilation provided a measurable clinical benefit, with significantly improved lung function, exercise capacity, and quality of life as compared with usual care.”4
Published in The New England Journal of Medicine4
The first prospective randomised controlled trial using Chartis® to select candidates for Zephyr Endobronchial Valve treatment.
Methods & Endpoints
- 68 patients were confirmed with the Chartis System to be collateral ventilation (CV) negative and likely responders to Zephyr® therapy, and randomised 1:1 to either Zephyr Valve therapy or medical management.
- Primary outcome measures were differences between groups for changes in FEV1, FVC, and 6-minute walk distance (6MWD) from baseline to 6 months.
Study Design
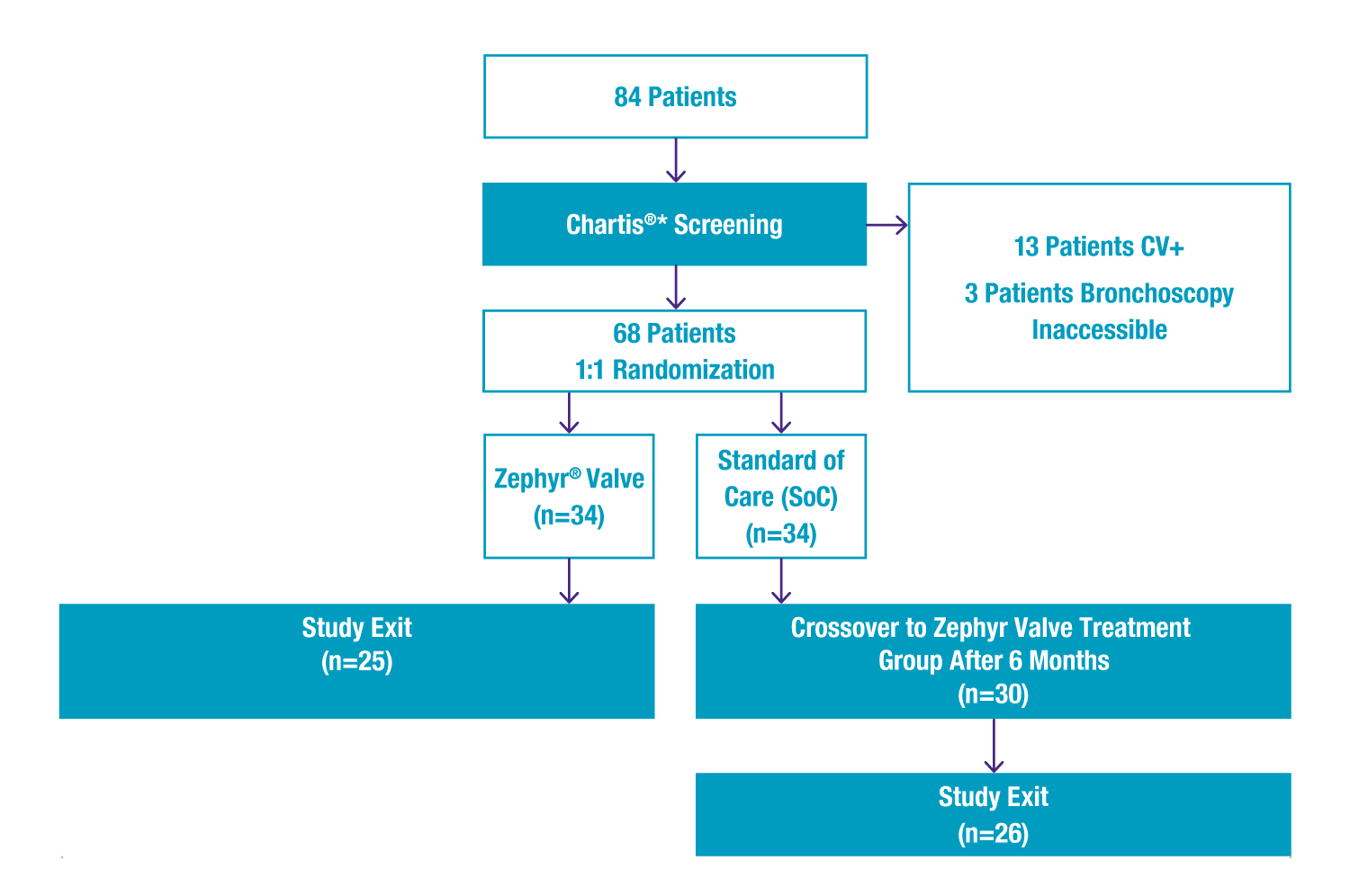
*The STELVIO trial was performed as an independent physician initiated and conducted trial. Pulmonx was not involved in any part of study.
Results
Primary Outcome in the Intention-to-Treat Population
Mean change from baseline to 6 months
FEV1
FVC
6MWD
| Zephyr Valve Treatment Group | SoC | Δ | |
| FEV1 | +20.9% | +3.1% | +17.8% |
| FVC | +18.3% | +4.0% | +14.4% |
| 6MWD | +19.6% | -3.6% | +23.3% |
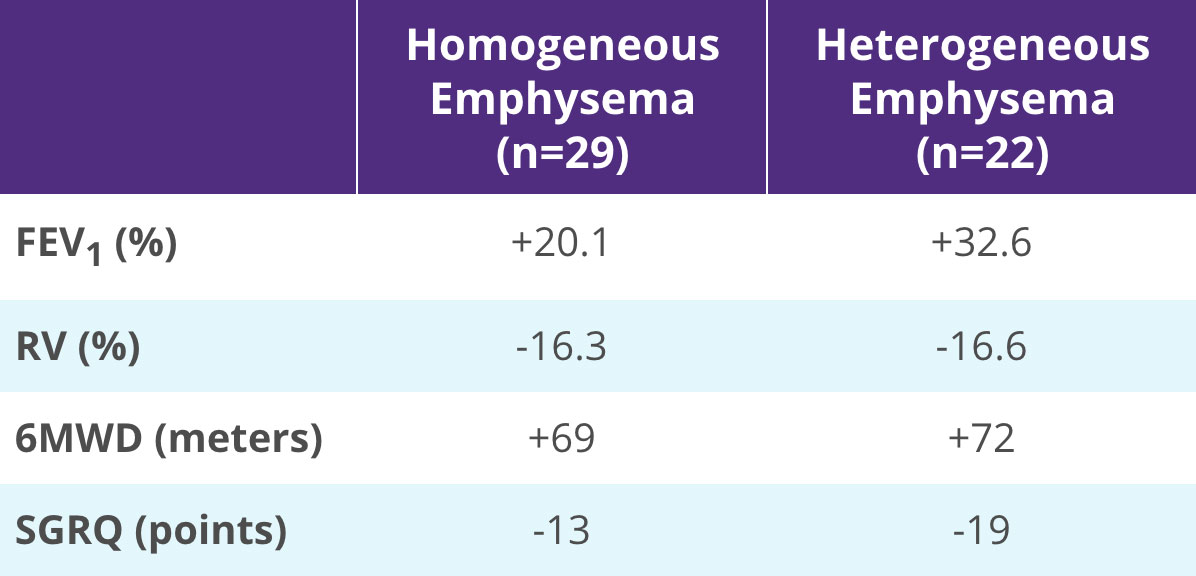
Clinical Benefits for Both Homogeneous and Heterogeneous Emphysema Subgroups
Effectiveness outcomes for all Zephyr Valve-treated patients who completed the study. Mean change from baseline.
Significantly Greater Responder Rates for Zephyr Valve-treated Patients than Standard of Care
MCID responder results among patients who completed the study.
Change in FEV1
Control Group
n=33
Zephyr Valve Group
n=25
Crossover Zephyr Valve Group
n=26
Change in 6MWD
Control Group
n=33
Zephyr Valve Group
n=25
Crossover Zephyr Valve Group
n=26
Change in SGRQ
Control Group
n=33
Zephyr Valve Group
n=25
Crossover Zephyr Valve Group
n=26
Conclusion
Endobronchial valve treatment significantly improved pulmonary function and exercise capacity in patients with severe emphysema characterized by an absence of interlobar collateral ventilation.
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.