
EBV therapy in selected patients with homogeneous emphysema without collateral ventilation results in clinically meaningful benefits of improved lung function, exercise tolerance, and quality of life. Given the very limited treatment options available for this patient population, EBV therapy should be considered in these patients.”3
Published in The American Journal of Respiratory and Critical Care Medicine3
The first prospective randomised controlled trial of Zephyr® Endobronchial Valves specifically in patients with homogeneous emphysema and little to no collateral ventilation (CV).
Methods
-
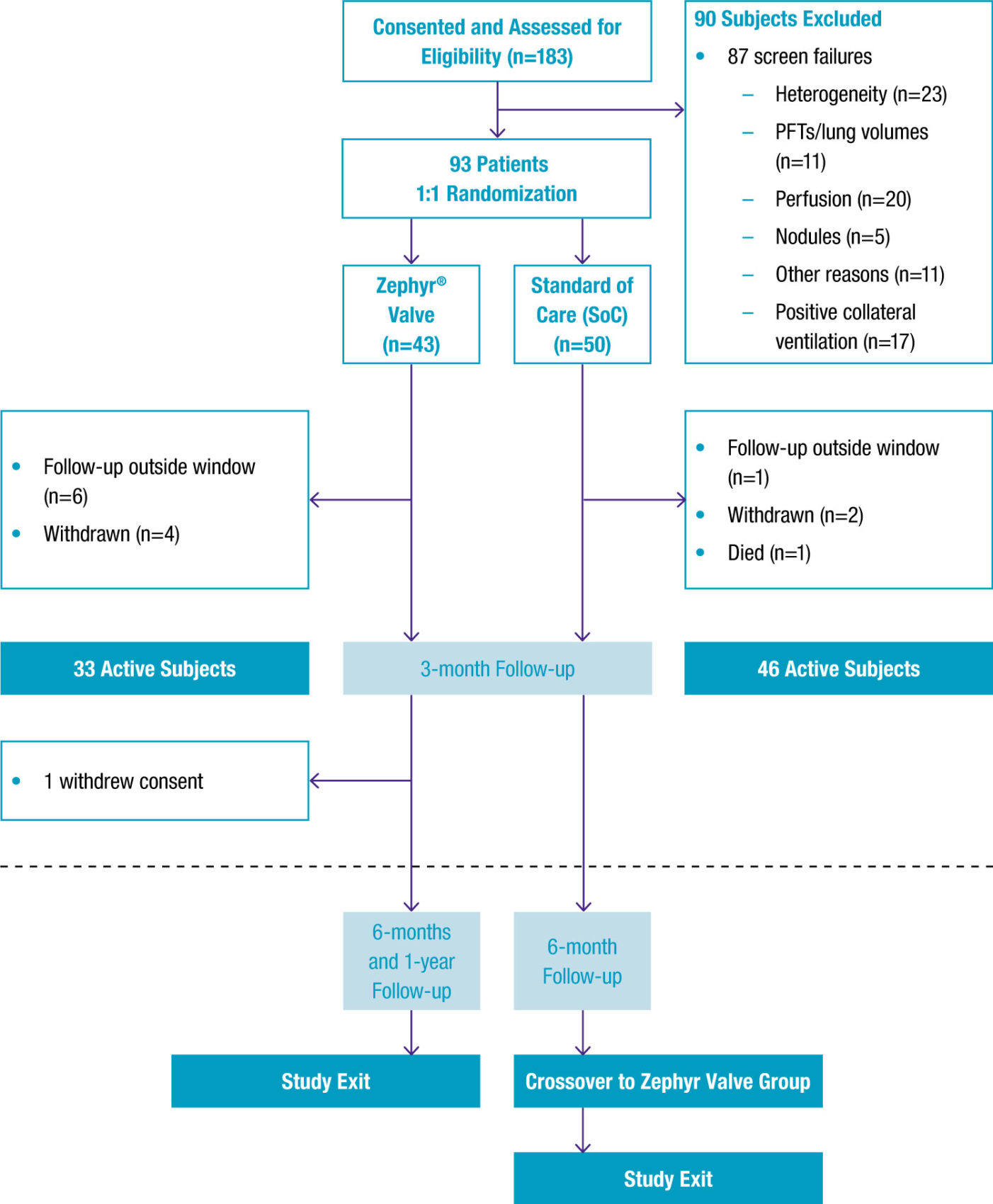
93 patients with homogeneous emphysema were confirmed with the Chartis® System to be CV negative and likely responders to Zephyr Valve treatment and randomised 1:1 to either Zephyr Valve treatment or medical management.
-
For Zephyr Valve-treated patients, target lobes were selected based on emphysema destruction scores and regional perfusion impairments and were then completely occluded with valves.
-
If patients did not feel a benefit, the valve position was assessed at 30 days by CT and removed and replaced if necessary.
Study Design
Results
Primary Outcome in the Intention-to-Treat Population
FEV1 Percent change from baseline to 3 months
Secondary Endpoints in the Intention-to-Treat Population
Zephyr Valve n=43, Control Group n=50
Change in 6MWD
Change in SGRQ
Change in RV
Change in FEV1
Change in mMRC Dyspnea Score
Conclusion
Patients with homogeneous emphysema can achieve clinically meaningful benefits in lung function, exercise tolerance, and quality of life with endobronchial valve treatment when they are pre-selected for absence of collateral ventilation and have complete lobar occlusion.
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.



