Zephyr® Endobronchial Valve
A proven treatment for breathlessness from severe COPD/emphysema
The Zephyr Endobronchial Valve is a clinically-proven bronchoscopic treatment for patients with severe COPD/emphysema who suffer from dypsnea despite optimized medical therapy.
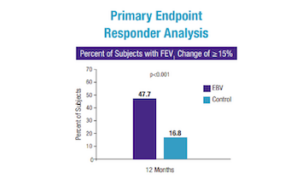
Patients treated with the Zephyr Valve compared to patients on medication alone were able to:1
- Return to a more active lifestyle
- Feel less shortness of breath
- Walk longer distances
- Have more energy
- Feel more confident leaving their home
Complications of the Zephyr Endobronchial Valve treatment can include, but are not limited to, pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.
Zephyr® Endobronchial Valve
A proven treatment for breathlessness from severe COPD/emphysema
The Zephyr® Endobronchial Valve is a clinically-proven bronchoscopic treatment for patients with severe COPD/emphysema who suffer from dypsnea despite optimized medical therapy.
Patients treated with the Zephyr Valve compared to patients on medication alone were able to:1
- Return to a more active lifestyle
- Feel less shortness of breath
- Walk longer distances
- Have more energy
- Feel more confident leaving their home
Complications of the Zephyr Endobronchial Valve treatment can include, but are not limited to, pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.