A Minimally Invasive Treatment Option for Patients with Severe COPD/Emphysema
With over 25,000 patients treated globally, the Zephyr Valve, FDA approved in 2018, is proven to help improve your patient’s lung function, exercise capacity and quality of life—without traditional surgery and many of its associated risks.20
If you treat patients with severe COPD and emphysema, we’re here to help.
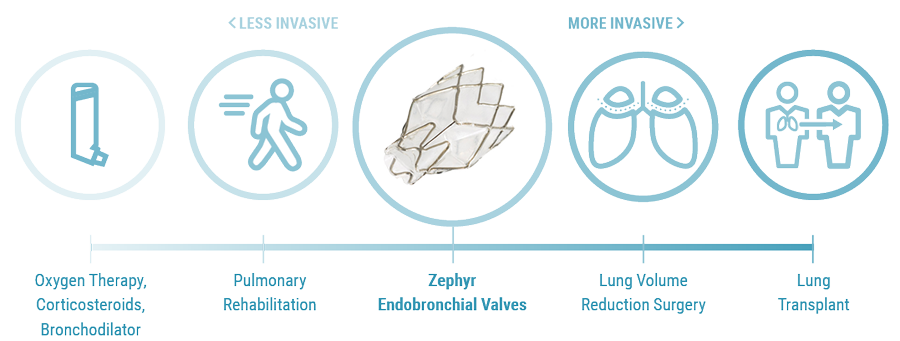
Initial Patient Selection
Patients who meet the following criteria should be evaluated for treatment with endobronchial valves:1-5
- Breathless despite optimal medical management (mMRC≥2)
- A confirmed diagnosis of COPD
- Non-smoking or willing to quit smoking
- Have an FEV1 ≤50% predicted
For more detailed information on clinical data and patient selection criteria please download our free resources.

GOLD 2020: Level A Evidence rating affirms that endobronchial valves, like the Zephyr Valve, are a viable, minimally invasive treatment option for severe emphysema, a form of COPD.
Indication and Mechanism of Action
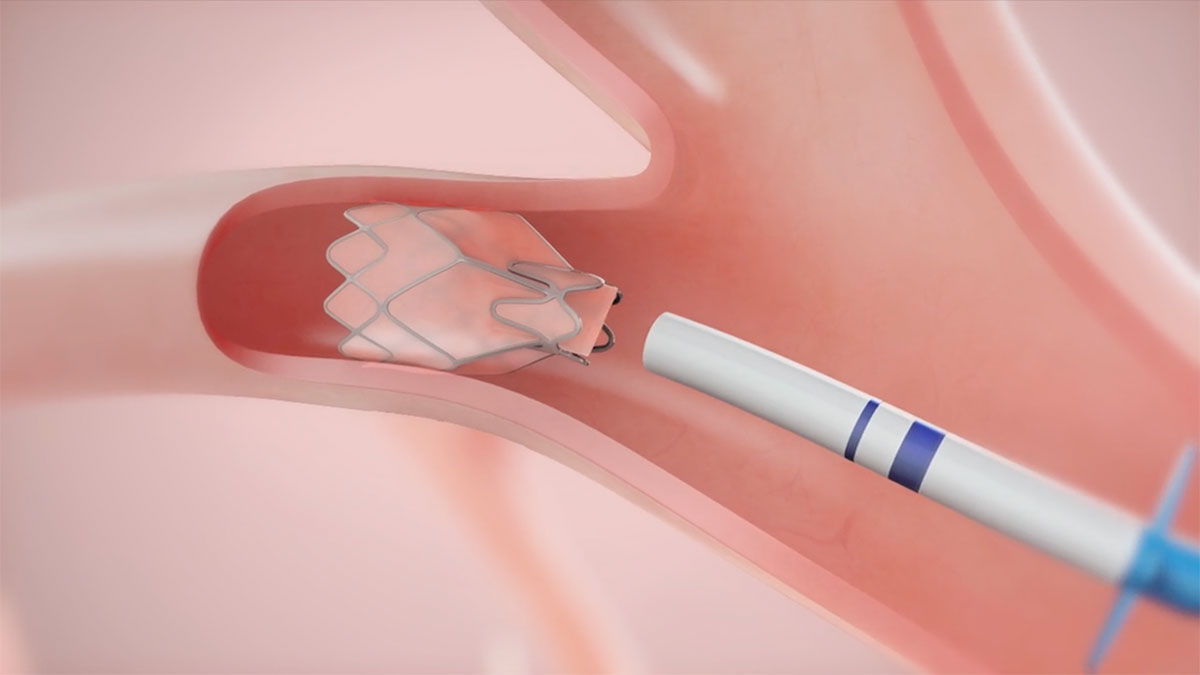
Endobronchial Valves are a new category of treatment for COPD and emphysema. The Zephyr Endobronchial Valve was approved by the FDA in 2018 and is indicated for the bronchoscopic treatment of patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation (CV). The Zephyr Valve is an implantable device used to occlude all airways feeding the hyperinflated lobe of a lung that is most diseased with emphysema.
These one-way valves comprised of nitinol and silicone allow trapped air to escape during exhalation, but close to prevent air from re-entering during inhalation. This allows atelectasis of the treated lobe and reduction in target lobe volume. The remaining lobes are then able to expand more fully and work more efficiently, reducing pressure on the diaphragm and improving overall lung function and breathing mechanics.1
If you’d like to learn more about how the Zephyr Endobronchial Valve
treatment can help your patients, our dedicated specialists are here to help.
Clinical Evidence
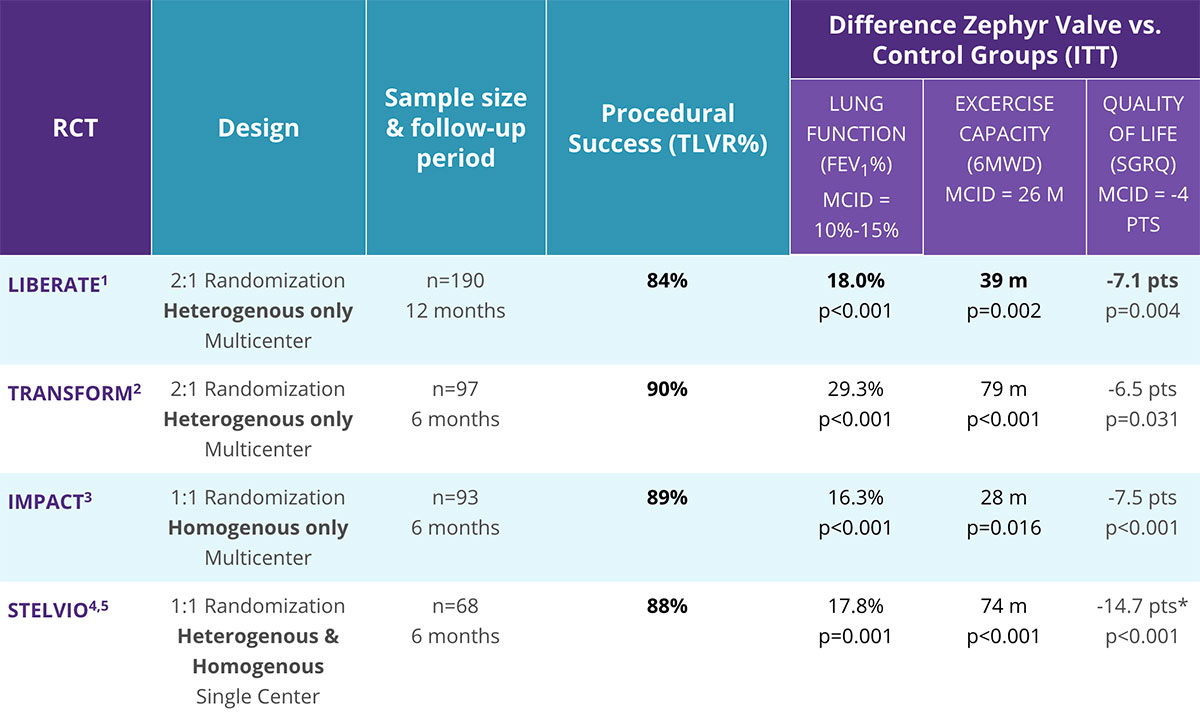
The Zephyr Endobronchial Valve Shows Clinically Significant Benefits in Lung Function, Exercise Capacity, and Quality of Life.
- 4 randomized clinical trials published using preselection tools to qualify appropriate patients1-5
- Published in the New England Journal of Medicine, The Lancet, and The American Journal of Respiratory and Critical Care Medicine1-5
- Statistically significant and clinically meaningful improvements compared to standard of care in multiple trials1-4
- GOLD 2020: Level A Evidence rating
- Treatment success seen in a broad range of primary and secondary endpoints1-5
If you have a patient with COPD that you would like to have evaluated for treatment with the Zephyr Valve,
we can connect you with a Zephyr Valve trained physician.
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.
25,000 People Have Been Treated with the Zephyr Valve
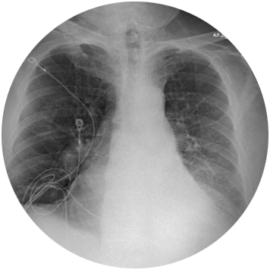
73-year-old Male Patient
Significant improvement in FEV1, decrease in TLC and RV, and increase in 6MWD
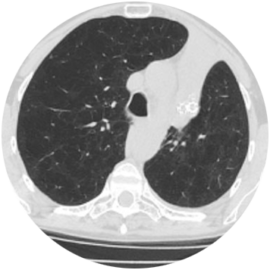
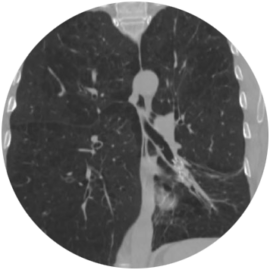
55-year-old Female Patient
Significant improvement in RV and FEV1, decrease in TLC, and increase in 6MWD
Find a Zephyr Valve Treatment Center

© 2021 Pulmonx Corporation or its affiliates. All rights reserved. All trademarks are property of their respective owners. GLO-EN-787-v5
Privacy Policy | Terms of Use | Sitemap | Prescriptive Information | In The News | Contact Us