Clinical Evidence
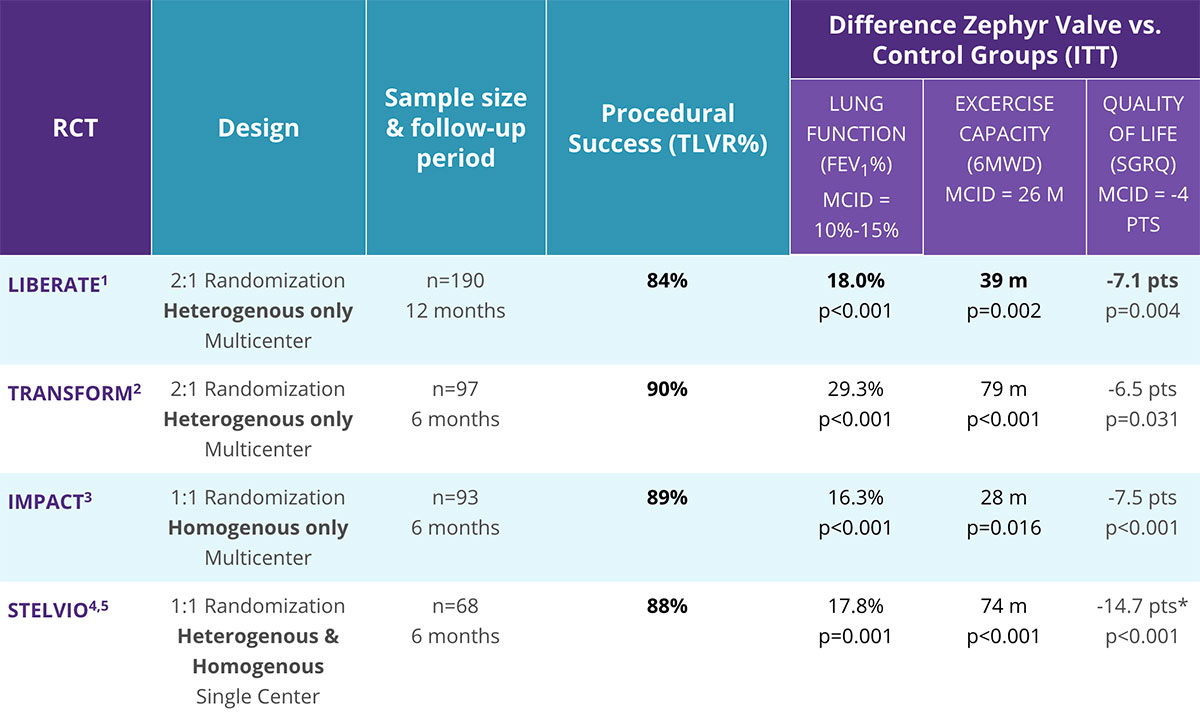
The Zephyr® Endobronchial Valve delivers clinical benefits — without surgery and its associated risks.1-5 Zephyr Valve is the most studied endobronchial valve and has consistently been shown to be a safe and effective treatment for patients with severe COPD/emphysema.
Recent Clinical Data
2023 GOLD Report Summary
The 2023 GOLD Report highlights patient benefits resulting from Endobronchial Valve treatment.
Study on Improved Survival
A single-center, retrospective study that found improved survival of 1.7 years after Zephyr Valve treatment.
US-EN-1834-v1